The second post bringing the exciting world of pathophysiology to light. Today, we’ll be learning about sickle cell anemia.
Disclaimer: I’m not a doctor – just a bioengineer who happens to find pathophysiology fascinating. If you find an error, please let me know! Als0, NONE of my drawings are to scale.
Sickle cell anemia is the ultimate morality tale about the importance of the little guy: in the 3 billion letters that make up your genome, one letter determines if you’re healthy or sick. Inheriting the wrong gene for hemoglobin can cause extremely painful episodes, recurring infections, and even death. Let’s see how it happens.
It all starts with what scientists like to call the Central Dogma of Biology. It says that information comes from your DNA, gets translated into amino acids, which get strung together to form proteins. DNA is the instructions, amino acids are like LEGOs, and the proteins are like the resulting LEGO dinosaur.

The central dogma of biology: DNA provides the instructions for the order of amino acids (LEGOs), which fold to create a protein (or dinosaur).
Changing the DNA can have a lot of different effects. Sometimes, it does almost nothing – you use a blue block instead of a green one, but the dinosaur still works the same way. However, sometimes changing just one letter can have disastrous consequences: you grab a 4-length brick instead of a 2-length brick, and now nothing fits together properly.
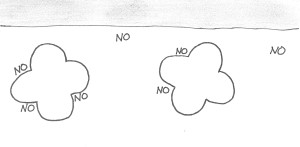
The mutation that causes sickle cell anemia comes from a single letter change in the DNA. In this case, the letter tells the body to use a sticky amino acid (valine) instead of the normal one (glutamic acid) in one part of the hemoglobin molecule. Now, hemoglobin’s job is to carry oxygen to your cells. To do that, it has one shape when it’s carrying oxygen and another shape when it has dropped the oxygen off. When a mutated hemoglobin has no oxygen, the sticky amino acid is exposed, and just happens to perfectly stick to another amino acid on the outside of another hemoglobin molecule.
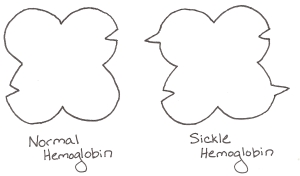
Hemoglobin is a four-lobed protein. Sickle hemoglobin has a sticky amino acid on two lobes… that perfectly fits with two holes present on both normal and sickle hemoglobin.
These two amino acids stick together like Velcro, and other hemoglobin molecules start sticking to either end, causing a long chain of hemoglobin molecules to form inside the cell.
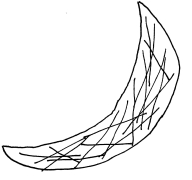
The molecules form long strings, which cause the red blood cells (which carry hemoglobin) to bend in the characteristic sickle-like shape (named after the sickle it resembles – note that there is no such thing as a hammered red blood cell). Changing that one amino acid to a stickier one is all the difference needed to create a debilitating disease.
To understand why that’s a problem, let’s say hello again to Jill – a girl living with sickle cell anemia.
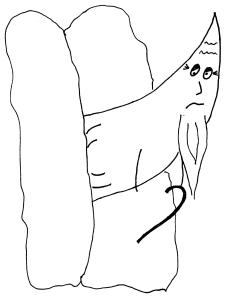
She’s going about her day – nothing unusual. Maybe she gets a little chilly, or maybe she hasn’t had much water. Something triggers the sickle hemoglobin molecules to stick together in her capillaries and veins. The molecules form long chains inside her cells. The red blood cells become hard and pointy, and they get stuck in the tiny capillaries that they would normally be able to squeeze through. These stuck cells prevent anything else from getting through the capillary, and Jill’s muscles and other tissues start to starve for lack of oxygen. (This effect is very similar to what happens during a heart attack or stroke.) The crisis is very painful as Jill’s body cries out for the oxygen it needs.

Blood is flowing from left to right in this vessel. The sickled cells block blood from getting to the lower capillary.
At this point, doctors can’t do much for Jill. They’ll provide her with pain medicine until her body can resume normal blood flow. In especially severe crises, doctors might give Jill a blood transfusion from someone with normal hemoglobin to help her body get oxygen to where it needs to go.
At this point, you might ask, “Where does the word “anemia” in sickle cell anemia fit in?” (Anemia refers to a low concentration of hemoglobin molecules in the blood.) To understand, let’s journey to the spleen – that mysterious organ that’s never quite described when you’re learning about the body. One of the spleen’s functions is to filter out old red blood cells. In a normal person, red blood cells start to deteriorate after about 120 days. At that point, they’re not as flexible as they used to be, and they can’t squeeze through the spleen’s tiny capillaries. The spleen grabs the old red blood cells out of circulation and recycles their iron.
Unfortunately, in patients with sickle cell, all the red blood cells act “old.” The repeated cycles of the hemoglobin molecules forming long chains inside the cell and then breaking up again makes the outside of the cell very stiff. After only 10 or 20 days of circulating around the body, these stiff, sickled cells get caught in the spleen and killed. This high rate of turnover means that the body just can’t produce enough red blood cells to keep up, and the patient remains in a stable state of anemia.
The spleen suffers in this bargain, too. Just like we saw above, sickled red blood cells can block the capillaries of the spleen. In fact, these capillaries get blocked more often than those anywhere else in the body because they are so small. Eventually, parts of the spleen start to die from lack of oxygen. That’s a problem: the spleen’s other function is to help fight bacteria by storing white blood cells. When those parts of the spleen die, the body is less able to fight off invading bacteria. In Africa, up to one half of untreated children with sickle cell die before they turn 5 because of this reduced ability to fight infection.
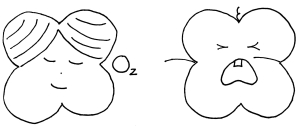
In some cases, a red blood cell gets so stiff that it breaks apart inside the blood vessel long before it gets to the spleen. The hemoglobin molecules are now floating free and bumping up against the blood vessel wall. These free hemoglobins gobble up molecules of nitric oxide. That’s a problem, because the body uses nitric oxide as a messenger to tell the blood vessels to open up wider. Without it, the vessels stay constricted, which sets the body up for another painful crisis like the one Jill just experienced.
What can we do? There are actually a lot of inventive ways to combat sickle cell. Children are often given antibiotics until they’re 5 years old to help fight off life-threatening infections like pneumonia. Patients experiencing painful crises, like Jill, can take pain medications, supplemental oxygen, or blood transfusions from someone with normal hemoglobin.
Interestingly, babies are not affected by sickle cell anemia. Fetuses and infants produce a special type of hemoglobin called, appropriately, fetal hemoglobin (HbF). This fetal hemoglobin grabs on to oxygen much tighter than an adult’s hemoglobin does so that the baby can grab oxygen from its mother’s blood.
Infants continue to produce HbF until they’re about 6 months old, when their adult hemoglobin (normal or sickled) takes over. Researchers have found certain drugs (hydroxyurea) that trick the body into continuing to produce fetal hemoglobin well into adulthood. For patients with sickle cell anemia, this extra production of a normal hemoglobin is enough to banish most of their painful symptoms.
The story of why sickle cell disease has persisted through the generations instead of dying out is a fascinating one – and one that will be saved for a later post. For now, let’s remember the power of the little guy, for good or evil.
Sources/Further Reading:
- Platt et al., Hydroxyura enhances fetal hemoglobin production in sickle cell anemia. Journal of Clinical Investigations. 1984; 74(2): 652-656.
- Human Genome Project.
- Ashley-Koch et al., Sickle Hemoglobin (Hb S) Allele and Sickle Cell Disease: A HuGE Review. American Journal of Epidemiology. 1999; 151(9): 839-845.
- Redding-Lallinger and Knoll. Sickle Cell Disease – Pathophysiology and Treatment. Current Problems in Pediatric and Adolescent Health Care. 2006; 36: 346-376.